
Polymerization is the process of combining many small molecules known as monomers into a covalently bonded chain. During the polymerization process, some chemical groups may be lost from each monomer. The distinct piece of each monomer that is incorporated into the polymer is known as a repeat unit or monomer residue.
Laboratory synthetic methods are generally divided into two main categories, step-growth polymerization and chain-growth polymerization. The essential difference between the two is that in chain growth polymerization, monomers are added to the chain one at a time only, whereas in step-growth polymerization chains of monomers may combine with one another directly]. However, some newer methods such as plasma polymerization do not fit neatly into either category. Synthetic polymerization reactions may be carried out with or without a catalyst.

Atomic force microscopy (AFM) is a powerful technique for investigating surfaces. In the imaging mode, a sharp tip is scanned over a surface and some surface parameter monitored. Traditionally, the deflection of a cantilever holding the tip is monitored by a photodiode. When the tip scans across the surface, it will interact with the latter, and deflect according to the interaction: if there is an attractive interaction between the surface and the tip, the tip will be deflected towards the surface, whereas deflection away from the surface will occur in the case of a repulsive tip-surface interaction. In the most frequently used AFM technique, the deflection is kept constant through a feed-back loop, and the z-movement of the stage needed for accomplishing this monitored. From this, one essentially obtains an interaction map, which under certain conditions may be translatable to a topographic map.

Dynamic Mechanical Analysis (abbreviated DMA, also known as Dynamic Mechanical Spectroscopy) is a technique used to study and characterize materials. It is most useful for studying the viscoelastic behavior of polymers. A sinusoidal stress is applied and the strain in the material is measured, allowing one to determine the complex modulus. The temperature of the sample or the frequency of the stress are often varied, leading to variations in the complex modulus; this approach can be used to locate the glass transition temperature of the material, as well as to identify transitions corresponding to other molecular motions.

Differential scanning calorimetry or DSC is a thermoanalytical technique in which the difference in the amount of heat required to increase the temperature of a sample and reference is measured as a function of temperature. Both the sample and reference are maintained at nearly the same temperature throughout the experiment. Generally, the temperature program for a DSC analysis is designed such that the sample holder temperature increases linearly as a function of time. The reference sample should have a well-defined heat capacity over the range of temperatures to be scanned.

Thermogravimetric analysis or thermal gravimetric analysis (TGA) is a type of testing that is performed on samples to determine changes in weight in relation to change in temperature. Such analysis relies on a high degree of precision in three measurements: weight, temperature, and temperature change. As many weight loss curves look similar, the weight loss curve may require transformation before results may be interpreted. A derivative weight loss curve can be used to tell the point at which weight loss is most apparent. Again, interpretation is limited without further modifications and deconvolution of the overlapping peaks may be required.
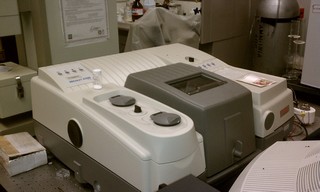
Fourier transform infrared spectroscopy (FTIR) is a technique which is used to obtain an infrared spectrum of absorption, emission, photoconductivity or Raman scattering of a solid, liquid or gas. An FTIR spectrometer simultaneously collects spectral data in a wide spectral range. This confers a significant advantage over a dispersive spectrometer which measures intensity over a narrow range of wavelengths at a time. FTIR technique has made dispersive infrared spectrometers all but obsolete (except sometimes in the near infrared) and opened up new applications of infrared spectroscopy.
The term Fourier transform infrared spectroscopy originates from the fact that a Fourier transform (a mathematical algorithm) is required to convert the raw data into the actual spectrum.

As a technique, GPC or SEC was first developed in 1955 by Lathe and Ruthven. The term gel permeation chromatography can be traced back to J.C. Moore of the Dow Chemical Company who investigated the technique in 1964. It is often necessary to separate polymers, both to analyze them as well as to purify the desired product. When characterizing polymers, it is important to consider the polydispersity index (PDI) as well the molecular weight. Polymers can be characterized by a variety of definitions for molecular weight including the number average molecular weight (Mn), the weight average molecular weight (Mw), the size average molecular weight (Mz), or the viscosity molecular weight (Mv). GPC allows for the determination of PDI as well as Mv and based on other data, the Mn, Mw, and Mz can be determined.

Rheometer is a laboratory device used to measure the way in which a liquid, suspension or slurry flows in response to applied forces. It is used for those fluids which cannot be defined by a single value of viscosity and therefore require more parameters to be set and measured than is the case for a viscometer. It measures the rheology of the fluid.
There are two distinctively different types of rheometers. Rheometers that control the applied shear stress or shear strain are called rotational or shear rheometers, whereas rheometers that apply extensional stress or extensional strain are extensional rheometers. Rotational or shear type rheometers are usually designed as either a native strain-controlled instrument (control and apply a user-defined shear strain which can then measure the resulting shear stress) or a native stress-controlled instrument (control and apply a user-defined shear stress and measure the resulting shear strain).

Many materials (ex. soil, sand, packed catalyst beds) consist of a large number of particles or fibers packed closely together. In between the solid particles or fibers there is open space, giving rise to pores through which fluids can flow. An object does not have to consist of many particles to be porous; for instance, it could simply be composed of a single continuos solid body that has many pores (or holes) in it. Such is the case with certain rocks and filters. Regardless of how the porous medium is constructed, because of the irregular, tortuous nature of the pores it is exceedingly difficult to model fluid flow through such materials exactly.

A glovebox (or glove box) is a sealed container that is designed to allow one to manipulate objects where a separate atmosphere is desired. Built into the sides of the glovebox are gloves arranged in such a way that the user can place his or her hands into the gloves and perform tasks inside the box without breaking containment. Part or all of the box is usually transparent to allow the user to see what is being manipulated. Two types of gloveboxes exist: one allows a person to work with hazardous substances, such as radioactive materials or infectious disease agents; the other allows manipulation of substances that must be contained within a very high purity inert atmosphere, such as argon or nitrogen. It is also possible to use a glovebox for manipulation of items in a vacuum chamber.

Ultraviolet-visible spectroscopy or ultraviolet-visible spectrophotometry (UV-Vis or UV/Vis) refers to absorption spectroscopy in the ultraviolet-visible spectral region. This means it uses light in the visible and adjacent (near-UV and near-infrared (NIR)) ranges. The absorption in the visible range directly affects the perceived color of the chemicals involved. In this region of the electromagnetic spectrum, molecules undergo electronic transitions. This technique is complementary to fluorescence spectroscopy, in that fluorescence deals with transitions from the excited state to the ground state, while absorption measures transitions from the ground state to the excited state.